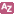The ProkaryotesProkaryotes have rigid cell walls, which preclude life as predators. They are restricted to life as chemists, and do their metabolism via transport and chemistry. This is in marked contrast to the eukaryotes, which are capable of engulfing (by a process called phagocytosis) other cells, and thus engaging in biology. In essence, the prokaryotes spurn life as biologists in order to optimize their skills as chemists. The full effect of such evolution is now easily visible through the genomic analyses of prokaryotes, where, in general, high percentages of the structural genes are involved with membrane and transport processes. In many cases, up to 25% or more of the total genome deals with the
interface between the cell surface and the environment and is involved with
uptake, transport, or metabolism of environmental chemicals. In eukaryotes on the other hand, much of the
DNA is devoted to the more biological concerns such as development, regulation,
and differentiation. Finally, the
prokaryotes are metabolically very diverse, while the eukaryotes are quite
restricted in their abilities. The
prokaryotes have developed a metabolic repertoire that allows them to utilize
almost any energetically useful chemical available that is abundant on the
Earth.
Figure 6. Sources of energy and oxidants on Earth. Some of the energy sources available to organisms on Earth are shown on the left, with the most energy rich at the top and the least energy rich at the bottom. On the right are shown the available oxidants for the burning of these biological fuels. The fuels and oxidants commonly used by the eukaryotes are glucose and oxygen, while those available to the prokaryotes are HCOOH, CH2O, H2, H2S, CH4, S0, Fe++, NH4+, MN++, and CO2, SO4, FeOOH, NO2-/NH4+, NO3-/NH4+, MnO2, NO3-/N2 respectively. On the left one sees the potential energy sources, ranked from the most energy rich at the top to the least energy rich on the bottom. On the right are the oxidants that can be used to ‘burn” these fuels, with the best oxidant (oxygen) at the bottom, and the worst one (carbon dioxide) towards the top. Since a fuel needs to be ‘burned’ to yield energy, we can estimate the amount of energy available simply by connecting a given fuel with an oxidant. If the arrow connecting any given so-called redox pair slopes downwards, it indicates that energy is available from this combination, and there is almost certain to be one or more microorganisms capable of using this combination. In marked contrast, the eukaryotes utilize only a few organic carbon compounds, and only molecular oxygen as the oxidant- they sacrifice diversity for high-energy yield, while the prokaryotes occupy the diverse, lower energy habitats.
Contributed by: Dr. Kenneth Nealson |